Trending Assets
Top investors this month
Trending Assets
Top investors this month
$NARI Announces Randomized Controlled Trial
Investor Takeaway: this is another step towards Inari's goal of proving their devices result in better outcomes than catheter-directed thrombolysis which requires the use of thrombolytic drugs.
If the data proves out Inari's thesis, it could potentially increase the amount of pulmonary embolism "PE" patients treated which could create better outcomes for patients and significant growth opportunities for Inari.
From the press release:
“Historically, due to the major bleeding associated with lytic therapy, physicians needed to carefully weigh a patient’s risk of death against the risk of intervention, reserving advanced treatment for only the sickest of PE patients,” said Global Co-Principal Investigator, Dr. Carin Gonsalves, Professor of Radiology and Co-Director of the Division of Interventional Radiology at Thomas Jefferson University in Philadelphia, PA.
“By offering patients immediate symptom relief upon removal of significant clot burden without the risks of lytics, the potential for bloodless thrombectomy with the FlowTriever System has fundamentally altered the PE treatment landscape, challenging physicians to rethink risk stratification and the goals of intervention.”
“PEERLESS is the first-ever RCT to compare mechanical thrombectomy to CDT for the treatment of PE and aims to provide definitive data on interventional treatment options for these patients,” added Global Co-Principal Investigator, Dr. Wissam Jaber, PERT Director, and Director of the Cardiac Cath Lab at Emory University Hospital in Atlanta, GA. “The primary outcome for the trial is a hierarchical composite of outcomes including mortality, major bleeding events, clinical deterioration, and length of stay in the intensive care unit. These are highly relevant endpoints for patients and for the hospital systems that care for them.”
“With active engagement on over 30 investigator-initiated studies, and 1,000 patients currently enrolled in our three ongoing VTE registries – CLOUT, FLASH, and FLAME – our clinical pipeline is as robust as ever,” said Bill Hoffman, Inari’s Chief Executive Officer. “PEERLESS opens a new chapter in our clinical story, answering the calls of physicians around the world for randomized control data to inform guidelines and redefine VTE treatment pathways around the world.”
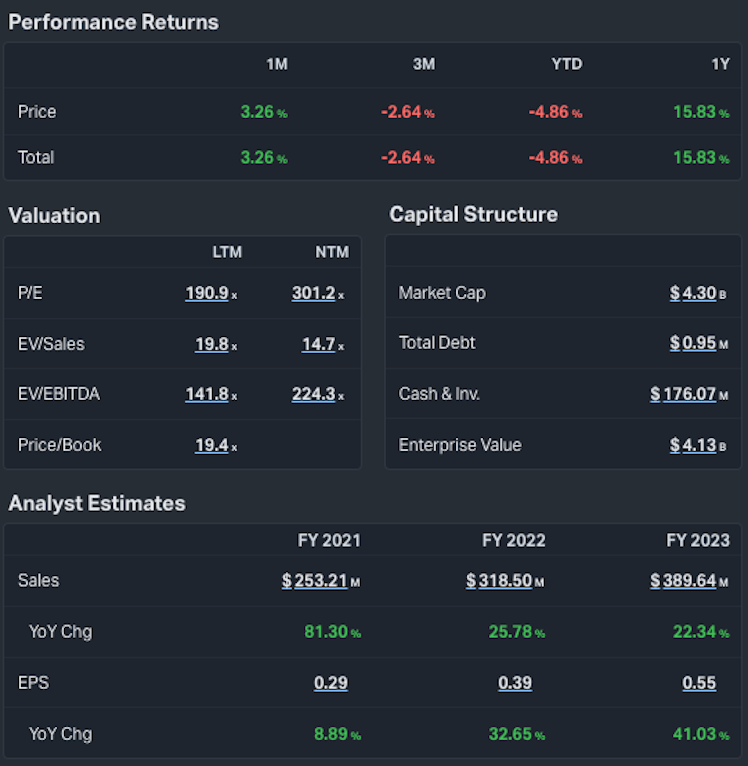
Koyfin Data:
Inari Medical, Inc.
Inari Medical Announces Randomized Controlled Trial Evaluating Clinical Outcomes of the FlowTriever® System in Pulmonary Embolism Patients | Inari Medical, Inc.
IRVINE, Calif., Oct. 18, 2021 (GLOBE NEWSWIRE) -- Inari Medical, Inc. (NASDAQ: NARI) (“Inari”) a medical device company focused on developing products to treat and transform the lives of patients suffering from venous diseases, announced planned enrollment of the PEERLESS trial.
Already have an account?